Gary Ades on Food Safety: HACCP! HACCP! HACCP! - PART 2

In Part 1 of this series, the background and five preliminary steps of HACCP were discussed. In this part we will discuss each of the seven principles of HACCP.
Table of Contents
Principle 1: Identify Potentially Hazardous Foods
Principle 2: Identify Critical Control Points (CCPs)
Principle 3: Establish Critical Limits
Principle 4: Establish Monitoring Procedures
Principle 5: Establish Corrective Actions
Principle 6: Establish Procedures for Verification
Principle 7: Establish Procedures for Recordkeeping
In Summary
 The National Advisory Committee On The Microbiological Criteria For Foods (NACMCF), the group as stated in Part 1 "that has had a significant impact on defining HACCP", defines HACCP as a systematic approach to be used in food production as a means to assure food safety. HACCP systems must be developed by individual producers and tailored to their individual food production, processing, packaging, distribution and retail requirements. While the principles of HACCP can apply to all hazards associated with food production, the NACMCF recommends against the use of HACCP for reasons other than assurance of food safety.
The National Advisory Committee On The Microbiological Criteria For Foods (NACMCF), the group as stated in Part 1 "that has had a significant impact on defining HACCP", defines HACCP as a systematic approach to be used in food production as a means to assure food safety. HACCP systems must be developed by individual producers and tailored to their individual food production, processing, packaging, distribution and retail requirements. While the principles of HACCP can apply to all hazards associated with food production, the NACMCF recommends against the use of HACCP for reasons other than assurance of food safety.
The purpose and the principles stated by the NACMCF in its August 14, 1997 document are:
Purpose: HACCP is a systematic approach to food safety consisting of seven principles:
Principles:
1) Conduct a hazard analysis. Prepare a list of steps in the process where significant hazards occur and describe the preventive systems.
2) Identify the critical control points (CCP) in the process.
3) Establish critical limits for preventive measures associated with each identified CCP.
4) Establish CCP monitoring requirements. Establish procedures for using the results of monitoring to adjust the process and maintain control.
5) Establish corrective action to be taken when monitoring indicates that there is a deviation from an established critical limit.
6) Establish procedures for verification that the HACCP system is working correctly.
7) Establish effective record-keeping procedures that document the HACCP system.
Principle 1: Identify Potentially Hazardous Foods
Hazard: Any biological, chemical or physical property that may cause food to be unsafe for consumption or may cause an unacceptable health risk to the consumer. (CODEX Def.: A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect.)
Risk: An estimate of the likelihood of a hazardous occurrence.
Severity: The seriousness or consequence of a hazard, should the consumer receive the food.
Preventive Measures: Actions and activities that can be used to prevent or eliminate a food safety hazard or reduce it to an acceptable level. Preventive measures include a wide variety of actions.
A hazard must be controlled if it is: 1) reasonably likely to occur and 2) if not properly controlled, it is likely to result as an unacceptable health risk to consumers.
The hazard analysis is the backbone of the HACCP plan. To establish a plan that prevents food safety hazards, it is crucial that all significant safety hazards and their control measures be identified.
Only those hazards that are deemed significant should be taken through a Critical Control Point (CCP) evaluation process (Decision Tree or other means). Significance is the level of risk a company is willing to take. This is an internal company decision that should be made with input from the highest levels. This is the place where you must do a lot of soul searching and make the "hard decisions". Remember, HACCP, like any other program of its type, is meant to minimize risk, nothing can assure zero risk.
Risk is determined using a likelihood (probability) and severity evaluation.

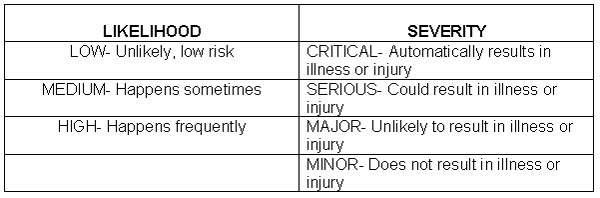
The company meets to decide the level of risk they are willing to take. This risk is assessed for the biological, chemical, and physical hazard of each ingredient and each step in the process.
Principle 2: Identify Critical Control Points (CCPs)
Control: (verb) To manage the conditions of an operation to maintain compliance with established criteria.
CCP: A step at which control can be applied and is essential to prevent, eliminate or reduce to acceptable levels a food safety hazard. A CCP must be controlled for the product to be reliably and consistently produced in a safe manner.
A CCP is a specific point in the process where application of a preventive measure effectively controls the hazard. For every significant hazard identified during the hazard analysis, there must be one or more CCPs where the hazard is controlled.
Points may be identified as CCPs when hazards can be prevented:
- Pathogen growth in a finished product can be prevented by control at the formulation or ingredient addition step. Pathogen growth can be controlled by cold storage and chilling the product.
Points may be identified as CCPs when hazards can be eliminated:
- Pathogens can be destroyed by cooking.
- Metal can be removed by in-line magnets.
Points may be identified as CCPs when hazards are reduced to acceptable levels:
- Foreign objects can be minimized by manual sorting and shaker tables.
Principle 3: Establish Critical Limits
Critical Limit: A value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate or reduce to an acceptable level the occurrence of a food safety hazard.
Control: (noun) The state in which correct procedures are being followed and criteria are being met.
Deviation: Failure to meet a critical limit.
Critical Limits are defined as the criteria that must be met for each preventive measure associated with a CCP. Critical Limits may be set for preventive measures such as temperature, time, humidity, moisture level, water activity, pH, acidity, salt concentration, available chlorine, or sensory characteristics such as odor, visual appearance or texture.
It may be necessary to consult scientific publications, regulatory guidelines or industry experts to determine appropriate critical limits. Tests may need to be conducted to identify critical limits for specific products or processes.
Examples of critical limits include:
- Pasteurizing milk at 161ºF for 15 seconds to destroy pathogens.
- Holding fresh seafood at or below 40ºF to control the growth of pathogens.
Principle 4: Establish Monitoring Procedures
Monitor: To conduct a planned sequence of observations or measurements to assess whether a CCP is under control and to produce an accurate record for future use in verification.
Monitoring is the process an operator relies upon to maintain control at a CCP. Assignment of the responsibility for monitoring is an important consideration. Monitoring indicates when there is a loss of control at a CCP and a deviation from the critical limit has occurred. When this happens, the critical limit is compromised and a corrective action must take place.
Monitoring also provides documentation that products were produced in compliance with the HACCP Plan. This information is needed for verification of the HACCP Plan (Principle 6).
Principle 5: Establish Corrective Actions
Corrective Action: Procedures to be followed when a deviation or failure to meet a critical limit occurs.
When critical limits are violated at a critical control point, the predetermined corrective actions must be instituted. Corrective actions should state procedures to restore process control and determine the safe disposition of the product. Corrective actions must be in place to: 1) determine disposition of the product; 2) correct the cause of the problem; and 3) maintain records of the corrective action.
Corrective actions may include:
- Destroying product
- Reprocessing product
- Rejecting raw materials
- Isolating product and evaluating for safety
Corrective actions are usually written in an "if-then" format. A separate file should be kept for all deviations and corresponding corrective actions.
Principle 6: Establish Procedures for Verification
Verification: The use of methods, procedures or tests, in addition to those used in monitoring, that determine if the HACCP system is in compliance with the HACCP Plan and/or whether the plan needs modification and revalidation.
Having a carefully documented, professionally designed HACCP Plan that includes all the necessary elements does not ensure the plan's effectiveness. Verification is method of assessing the effectiveness of the Plan and confirming that the system is working to produce safe food.
Verification audits, both internal and external, are an important component of the Principle of Verification. Audits are systematic evaluations that include on-site observations as well as review of records. It is best to have a verification audit performed by educated, knowledgeable, unbiased people who are not responsible for performing monitoring activities.
Verification audits should occur at a frequency that guarantees that the HACCP Plan is working properly. Verification must take place when there has been a change in the process, addition or replacement of equipment, modification in the product formulation and/or addition or replacement of a significant ingredient.
Principle 7: Establish Procedures for Recordkeeping
Accurate recordkeeping is a vital component of an effective HACCP Plan. Records provide documentation that critical limits have been monitored and met and that appropriate corrective action was taken when the critical limits were violated.
Some of the records kept as part of the HACCP system include:
- The HACCP Plan and supporting documentation used in developing the plan (i.e., hazard analysis worksheet, literature reviews, documentation of studies to determine critical limits, etc.)
- Monitoring records for CCPs
- Corrective Action records
- Verification records
It is also advisable to include in the HACCP Plan the supporting documentation created with the 5 Preliminary Steps: HACCP Team; Description of the Final Product; Identification of the Intended Use and Consumers; and Verified HACCP Flow Diagram with CCPs identified.
HACCP is an understandable, common sense approach to food safety. However, it cannot be bought "canned" and attached to the current food safety system. Each HACCP Plan is unique to the product or process for which it is written. Important prerequisites to the development and implementation of a HACCP program are the condition of the facility and the status of the systems currently utilized to produce food. The facilities and systems must be acceptable so not to compromise the goals of HACCP -- to produce food safe for consumers. Good sanitation procedures need to be in place. Employees must be properly educated in food hygiene principles and must have the knowledge necessary to do what is expected of them under a HACCP program. The facility and equipment must be conducive to safe food production.
The importance of planning and implementation of any food safety plan cannot be overstated. However, of equal importance is the commitment of company management to dedicate the time, personnel and finances to support the food safety system. As HACCP pioneer Dr. Howard Bauman stated:
"HACCP is a cooperative program and should involve everyone from the CEO to the newest employee in the plant. Everyone should be receiving training in the HACCP concept so that it is fully understood. Personnel used to train plant employees must have an extensive knowledge of the program. An overall trust must be developed in the company, since under the HACCP system people on the line have a responsibility to monitor CCPs and take corrective action if a problem occurs."
When fully supported by management and properly implemented, HACCP can be an effective and successful tool in producing safer, cleaner and higher quality foods in all areas of the food industry.
Gary Ades, Ph.D, is president of Technical Food Information Spectrum, Inc. (TFiS), a leading food safety and quality consulting firm. Dr. Ades is a professional food scientist and consultant with over 25 years of experience in the food industry. For more information, Dr. Ades can be reached at 800.248.8347 or via E-mail at tfis@ix.netcom.com.
